|
| |
Do you know how a moth emerges from its
cocoon, and how snake venom causes hemolysis ?
What if we apply the same principle in a
controlled manner, we then can dissolve all those fibrins which bind the blood
cells that formed the clots, so the clot is also dissolved ........
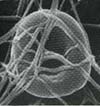
Fibrin filaments wrap around and entrap a single red blood cell. |
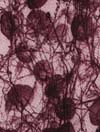
The beginning of a blood clot:
Platelet and Red blood cells become trapped in a
network of fibrin cables. |
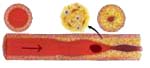
A floating time bomb, an emboli could be trapped
and blocking artery at any time. |
An all natural fibrinolytic ( Clot-dissolving ) agent
--- M-boless
Currently, the approved treatments for
strokes are Urokinase, Streptokinase
and t-PA.
(In
June 1996, the U.S. Food and Drug Administration approved the use of
intravenous t-PA for acute ischemic stroke. Approval was based on the
findings of a clinical trial sponsored by the National Institute of
Neurological Disorders and Stroke (NINDS) All three are
available in the form of intravenous infusion only. To work better, it must be given within three hours after the
onset of the attacks.
|
|
M-boless is an ORAL self-limiting formula that acts on t-PA ( Tissue Plasminogen Activator ), sort of pre t-PA
factor, but slower releasing.
t-PA activates plasminogen to generate plasmin which is a specific fibrinolytic enzyme in our normal homeostasis
procedures to dissolve fibrins that bind the blood cells in the formation of blood clots and other vascular deposits
(clots, plaque fragments etc..)
Traveling clots became thrombosis, or embolus in the blood stream, which may cause
blockages, thus cause strokes (CVA), Myocardial infraction (MI), Deep vein thrombosis (DVT), pulmonary embolism etc.
M-boless does not prolong normal homeostasis as were by NSAID, Heparin, and warfarin etc......M-boless does not prolong normal homeostasis as were by
NSAID, Heparin, and warfarin etc....
M-boless is activated only when fibrin is present
(clot forming), in normal
conditions it does not react with normal plasma proteins i.e. fibrinogen
(precursor of fibrin) or prothrombin, therefore it does not cause prolonged bleeding as
were treated by other less specific treatments, or non steroid anti-inflammatory
drugs (NSAID). i.e.. Heparin, warfarin, EDTA, aspirin, niacin etc...
M-boless's
fibrinolytic function peaks in 5-30 minutes
after the intake of M-boless and returns to normal level in 90
minutes.
It is a self limiting enzyme, it
is a safer, effective
alternative management for strokes, heart attacks, and thrombosis.
Its
clinical effective rate at over 90 %.
Some of t-PA's Clinical Indications:
- Lysis the emboli in pulmonary emboli.
- Lysis the emboli formed by unstable hemodynamics.
- Lysis thrombi obstructing coronary arteries.
- Prevents and treatment of ischemic stoke.
- Prevents and treatment of ischemic heart attack.
- Prevents forming of DVT, in high risk
populations.
- (Long period of sitting, sedentary life style,
narrow of diameter of arteries due to foreign materials
accumulation, stressful life styles.)
- Prevents forming of thrombosis in many surgical
procedures.
Precautions
associated with the use of I.V. infusion of t-PA
|
|
Active main ingredients of M-boless including :
M-boless Endopeptidase ......................................
Chelated-bioactivated biobran..........................
Plasmin : The active portion of the fibrinolytic system, an
enzyme with a high specificity for fibrin, with
the particular ability to dissolve formed fibrin clots but also
having a similar degradation effects on other plasma proteins and clotting factors
and proteins in general.
Urokinase: A substance found in the urine of mammals,
including human, and of other vertebrates, which activates the
fibrinolytic system, used in hospital for the treatment of
embolism. It decrease the levels of fibrinogen and plasminogen and
an increase in the amount of circulating fibrin may persist for 12-24
hours.
Other uses of t-PA and M-boless
Ophthalmology,
Neurology
|
|
In vitro Thrombolysis comparison
between M-boless and Urokinase
(Urokinase is current standard treatment in
hospitals, I.V. only)
In vitro artificial embolus at approx. 40 mg each; Placed in 37o
C; rotate at 9 rpm.
| M-boless
|
1250 unit |
125 unit |
62.5 unit |
| Total dissolved in
30 min. |
67% dissolved in 30 min. |
27.5% dissolved in 30 min. |
| |
91% dissolved in 120 min. |
|
| |
|
|
|
| Urokinase |
1250 unit |
300 unit |
60 unit |
| 19% dissolved in 30 min. |
|
|
| 58% dissolved in 60 min. |
|
|
| 90% dissolved in 120 min. |
60% dissolved in 120 min. |
32% dissolved in 120 min. |
|
In vivo Thrombolysis test in mice
Within 4 hours M-boless dissolved thrombosis from 24 mg to 2.5 mg.
M-boless does work, but we apply it in a milder and more controllable way in
human body.
| Route of intake |
Group |
Artificial Induced thrombosis
animal |
after 2-4 hrs. post M-boless |
P value |
| Animal # |
Average embolus weight (mg) |
| I.V. 1000 unit/kg |
Test |
10 |
7 |
2.5 + 1.3 |
P < 0.05 |
| Control |
11 |
9 |
24 + 6.8 |
| Intestine
6000 unit/kg
|
Test |
11 |
5 |
0.9 + 0.9 |
P < 0.01 |
| Control |
9 |
8 |
12.1 + 0.9 |
In vivo testing of Pulmonary
thrombosis in rabbits
- Use 125I embedded embolus injected in to Jugular vein as
induced pulmonary thrombosis.
- Use 2000 unit/kg and 600 unit/kg of M-boless through intestine.
- Blood samples were taken on 0.5, 1, 2, 3 and 5 hours.
- 125 I was measured, it appears marked increase of 125I
in 3 hrs, and increasing in 5 hr. which shows direct correlation of
amount of dissolved embolus with the dosage.
|
Treat with M-boless |
2000 unit /kg |
600 unit /kg |
Control |
| 125 I measured |
3hr |
Test |
73 + 12 |
67 + 13 |
58 + 8.7 |
| 125 I measured |
5hr |
Test |
71 + 11 |
73 + 18 |
53 + 12 |
| Toxicity
test of M-boless
Test 1:
40 mouse at body weight between 18-20 gram, 20 are male, 20 are
female, divided into mixture genders of 4 groups. I.V.
injection of M-boless for 7 days. The test determined LD50=144000
unit/kg.
Test 2:
80 Wister mouse, bodyweight between 160 to 250 gram, 50% in
each gender, divided into mixture of 4 groups, three groups
receive I.M. injection of 25% of LD50 dosage and
10% of LD50 dosage, once a day, for two weeks.
Control group of 20 mouse are injected with saline.
Measurement of GPT, BUN and Hematocrit and platelets count show no
change in either groups, indicates no kidney or liver damage was
induced.
Test 3:
12 Dogs, bodyweight between 10 to 17 kg. 50% gender each,
divided into mixture gender of 3 groups. Two groups receives I.V.
injection of 12.5% of LD50 dosage and 20% of LD50
dosage, once a day, for two weeks; Control groups received saline
injection. Group I, II receive 12.5% and 20% of LD50
dosage, experience lost control of urinary, and bowel movement,
vomiting, drowsiness, salivation, lost of balance with in one hour
immediate after the injection, symptom subsided after one hour after
injection. Group III does not show any symptom, as the control
group.
Pre-treatment and post-treatment measurement of blood
picture of GPT, BUN and Hematocrit and platelets count show no
change in either groups, indicates no kidney or liver damage was
induced.
|
Human clinical tests:
|
Summary of Phase I
M-boless capsule clinical trail
M-boless clinical test
Phase
1, 15 healthy
volunteers.
- Single and multiple oral intakes of M-boless, No adverse effect noticed.
- Single and multiple oral intakes of M-boless, No adverse effect to Liver,
Kidney functions, No adverse effect on
blood sugar level, No adverse effect on blood lipid level.
- Single and multiple oral intakes of M-boless,
Fibrinolytic and U-globulin dissolving time is shortened; No effect on
Bleeding time and Coagulation time.
|
|
Summary of Phase II
M-boless capsule clinical trail
1990 to 1991 total of 453 cases of cerebra-thrombosis were treated, 303 cases
were given M-boless, 150 cases were give placeboes, two capsules each time,
three times per day, for 21 days.
All 453 patients suffered from Carotid artery thrombosis, with various degree
of impair control of extremity with confirmation by CT of thrombosis
blockage.
| Age |
Male |
Female |
| Treatment group |
200 |
100 |
| Control |
101 |
49 |
| P value |
>0.05 |
>0.05 |
Comparison of Pre and Post treatment of Blood pictures and Globulin
dissolving time.
| |
# of cases |
Pre-treatment |
Post-Treatment |
P value |
| Fibrinogen (g/l) |
294 |
3.91 + 1.09 |
3.3 + 0.8 |
<0.001 |
| Globulin dissolve time |
203 |
215.1 + 83 |
176.8 + 65.8 |
<0.001 |
| Plasma viscosity |
222 |
1.83 + 0.2 |
1.73 + 0.15 |
<0.001 |
| Whole blood viscosity |
221 |
5.2 + 2.5 |
5.20 + 1.45 |
<0.001 |
| Hematocrit |
219 |
47.2 + 29.3 |
43.3 + 3.5 |
<0.05 |
| ESR |
217 |
22.45 + 11.45 |
21.08 + 11.1 |
>0.2 |
| Platelet aggregation |
182 |
59.9 + 22.5 |
53.80 + 16.9 |
>0.2 |
Discussion: Post treatment shows improvement in
Fibrinogen
volume, Globulin dissolve time, Viscosity of blood, Plasma viscosity,
Hematocrit, and Platelet aggregation.
The amount of improvement is determined by the schedules of Clinical
neurological damage classification 1-15 ( light), 16-30
(mid), 31-45 (heavy).
| |
Treatment group (%) |
Control group (%) |
| Total cured |
68 (22.4) |
10 (6.6) |
| Noticeable improved |
155 (51.1) |
32 (21.3) |
| Improved |
61 (20.1) |
61 (40.7) |
| No Change |
19 (6.3) |
38 (25.3) |
| Worse |
0 (0) |
8 (5.3) |
| Decease |
0 (0) |
1 (0.6) |
| Total effectiveness |
284 (93.7) |
103 (68.6) |
| Effective ratio |
223 (73.6) |
42 (28.0) |
Conclusion: M-boless its total effective at 93.7%, noticeable effective at
73.6%.
Conclusion:
Under the supervision of Health
Department, at the specified locations, Phase 1 and Phase 2 clinical tests have
proven that oral capsule of M-boless for three weeks have remarkable effect for
the condition of Ischemic cerebrovascular disease, paralysis caused by
stroke (CVA), and lost of speech
and limbs functions from the result of stroke.
There is no adverse effect to
liver function, kidney function, blood sugar level, blood lipid level. It is a
safe potential fibrinolytic agent.
Also during the test period, we
notice patientís fibrinogen level reduced to normal level, EU-globulin lysis
time (ELT) has shortened, whole blood viscosity and plasma viscosity are
noticeable reduced, and reduce the
platelet aggregation tendency which indicates oral intakes of M-boless is
beneficial to avoid heart and cerebral disease for the patients who are in high
risk of.
M-boless capsules, its
effectiveness is noticeable, no toxic side effects, it is in oral format easy
and convenient to use. Suitable to use in the prevention of any of embolism or
any of the blood thrombosis type of disorder.
For Acute stroke, lost of speech function and limb paralysis due to stroke have very noticeable effect.
It is very suitable and potential to use it in prevention of heart and
cerebral vascular disorders.
M-boless is not only for
therapeutic, it is also an idea agent for prevention.
|
|
Summary of Phase III
M-boless capsule clinical trail
CVA Cerebral Vascular
Accident is a very frequent brain vascular disorder. Its onset fast,
causes damage severely, it seriously affect patientsí quality
of life. Although, there are many treatments available for this type of
disorder, yet none of
any oral medication
provides such effective results. M-boless
is formulated by chelating several
micro-organism produced enzymes, they are mainly to activate
Plasminogen, and plasmin.
In 1990 phase II study of 450 plus patients, under double blind,
random test, proves that M-boless has 93.7% effective rate in the
treatment of Ischemic brain vascular disorder.
To be further verify the efficacy and if any possible adverse
effect of the formula. We
have organized these 16 hospitals under the guide line of
health department new drug application to conduct this phase III
study.
In 1992 to 1993 total of 16 hospitals and 1560 cases participated.
| |
# of cases |
Pre-treatment |
Post-Treatment |
P value |
| Fibrinogen (mg/l) |
1161 |
373.10 + 93.20 |
309.10 + 71.00 |
<0.01 |
| Euglobulin dissolve time |
1053 |
182.50 + 48.35 |
155.10 + 38.10 |
<0.05 |
| Plasma viscosity |
1074 |
1.79 + 0.18 |
1.67 + 0.16 |
<0.05 |
| Whole blood viscosity |
1074 |
7.41 + 1.40 |
6.64 + 1.14 |
<0.01 |
| Platelet aggregation |
981 |
66.80 + 13.25 |
57.10 + 11.43 |
>0.05 |
Total effective rate 88.21%, Noticeable effective
rate 68.91%
| Hospital |
# of cases |
Age |
Condition |
Days of treatment |
Noticeable improved |
Improved |
No Change |
Worse |
| M |
F |
L |
M |
H |
| 1 |
210 |
126 |
84 |
110 |
85 |
15 |
2.32 |
98 |
44 |
40 |
0 |
| 2 |
171 |
112 |
59 |
89 |
73 |
9 |
3.16 |
59 |
37 |
11 |
0 |
| 3 |
130 |
87 |
43 |
105 |
20 |
5 |
26.70 |
51 |
42 |
20 |
0 |
| 4 |
50 |
27 |
23 |
24 |
23 |
3 |
31.35 |
16 |
25 |
7 |
0 |
| 5 |
33 |
26 |
7 |
10 |
17 |
6 |
26.70 |
14 |
10 |
5 |
0 |
| 6 |
60 |
40 |
20 |
28 |
27 |
5 |
6.10 |
25 |
15 |
12 |
0 |
| 7 |
43 |
29 |
14 |
33 |
7 |
3 |
13.75 |
12 |
4 |
6 |
0 |
| 8 |
50 |
39 |
11 |
26 |
21 |
3 |
9.75 |
34 |
9 |
3 |
0 |
| 9 |
42 |
25 |
17 |
29 |
12 |
1 |
6.21 |
13 |
4 |
7 |
0 |
| Other hospitals |
771 |
490 |
281 |
485 |
244 |
42 |
4.05 |
372 |
111 |
73 |
0 |
| Total |
1560 |
1001 |
559 |
939 |
529 |
92 |
9.69 |
694 |
301 |
184 |
0 |
|
|
Recommended dosage: 2 caps. each time, three times per
day, take it 30 minutes before meals. 20 days as
a treatment cycle. Use 1, 2, or 3 cycles or until the disappearance of
the symptoms. Medical monitoring of Hematocrit, platelet count,
activated partial thromboplastin time, or prothrombin time is advised. Cautious
should be given to patients with active internal bleeding, history of
cerebrovascular accident, recent (within 2 months) intracranial or
intraspinal surgery, intracranial neoplasm, Arteriovenous malformation
or aneurysm, known bleeding diathesis, and uncontrolled arterial
hypertension.
Although hemorrhagic
strokes are far less common, (occur when an artery bursts), care should
be given to differentiate the type and the cause of the strokes and the
timing of using M-boless. For example to use it after the hemorrhaging
is over, and no further bleeding is anticipated.
|
Links: http://www.stroke.org/acute_treat.cfm
|